HIV and TB testing sites start COVID-19 research
17 June 2020 | Story Niémah Davids. Photo Pexels. Voice Lerato Molale. Read time 5 min.
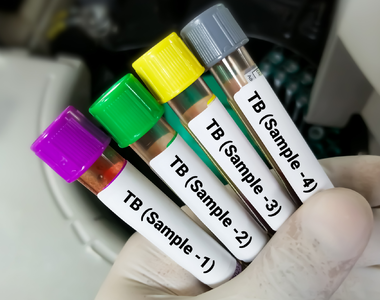
The Desmond Tutu HIV Foundation (DTHF) in association with the Desmond Tutu HIV Centre (DTHC), an accredited research centre at the University of Cape Town’s (UCT) Institute of Infectious Disease and Molecular Medicine (IDM), has been working to accommodate and incorporate COVID-19 research and trials at its existing HIV and tuberculosis (TB) research sites.
Since its inception in 2004, the DTHF has established several clinical research sites in under-resourced communities in South Africa, while focusing on communities at high risk of contracting HIV and TB. Through community partnerships and efforts, the foundation also collaborates with those most at risk in order to find innovative solutions to prevent and treat HIV, TB and other related infections.
To date, the foundation has expanded its clinical trial capacity throughout the country – this process has allowed scientists to conduct large community-based trials in HIV and TB, which include trials in vaccine development, prevention and treatment.
In February 2019 the DTHC Masiphumelele research site launched a new Aerobiology TB Research Facility, which focuses on the study of the transmission of TB organisms with a view to finding ways to halt the spread of the disease.
“We are already adapting our sites to be COVID-19 safe.”
Professor Linda-Gail Bekker, the DTHF’s chief operating officer, said in a recent interview that in light of the current COVID-19 pandemic, adapting the sites to incorporate research related to COVID-19 was essential.
“We are already adapting our sites to be COVID-19 safe. By this I mean improving the infection control measures so that we can include COVID-19-related research there,” she said.
“The infrastructure exists, so we can do this efficiently and affordably.”
Pivoting to include COVID-19 research
The renewed focus on COVID-19 research doesn’t mean HIV and TB clinical trials have taken a back seat at testing sites. On the contrary, Bekker stressed, work is continuing in earnest.
“With this new research addition, we are still able to continue the HIV and TB work we have been doing. This is critical for the well-being of the country long term, while at the same time pivoting to include COVID-19 product research,” she said.
Bekker explained that research related to COVID-19 at DTHF sites will focus on developing vaccines, monoclonals (forming a clone derived from a single individual or cell), pre-exposure prophylaxis, and therapeutics.
Behind the scenes, she said, ensuring that testing sites are COVID-19 safe and ready, and adhering to the National Department of Health’s strict guidelines, has been a top priority.
Stumbling blocks
But with new ventures come new challenges, she pointed out.
In this case, Bekker said, operations at testing sites came to a grinding halt when the COVID-19 pandemic hit the country. This as teams regrouped and tried to figure out the best way to move forward.
“We needed to restart our engines.”
To ensure sites were COVID-19 compliant, Bekker said spaces needed to be modified and extended accordingly, and in some cases, rebuilt.
“We are all moving forward. I think some [testing sites] are more ahead than others, but now we feel the urgency to get moving.”
Additions such as screens, outdoor tents, additional workspaces and more hand-washing stations were also made available.
“Emotionally, we also needed to get our heads right on how to do this, and if you like, to get back on the bike,” she said.
‘Moving forward’
Progress has been swift, Bekker added.
“We are all moving forward. I think some [testing sites] are more ahead than others, but now we feel the urgency to get moving,” she said.
“[COVID-19] proposals and products are coming in and need to be tested. At the same time, all of our HIV and TB work needs to continue, urgently, or delays will damage those processes.”
 This work is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License.
Please view the republishing articles page for more information.
Listen to the news
The stories in this selection include an audio recording for your listening convenience.