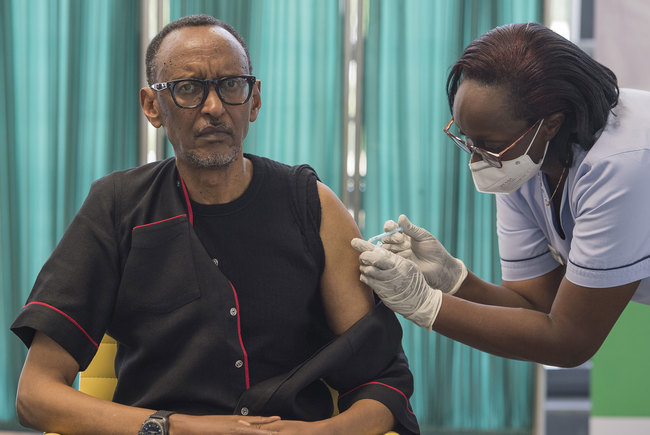
COVID-19 vaccines for African countries?
22 January 2021 | Story Benjamin Kagina. Photo Pexels / Sam Moqadam . Read time 6 min.
Vaccines for COVID-19 are generating a lot of talk. To shed some light on which vaccines are available for countries in sub-Saharan Africa, and how the process will work, The Conversation Africa’s Ina Skosana and Ozayr Patel asked Benjamin Kagina to answer a few questions.
What’s immediately available for African countries?
African health regulatory authorities must approve the use of vaccines before they can be rolled out on the continent. National health regulatory authorities around the continent are working closely with regional and global regulatory bodies to ensure the approval processes will be efficient.
The AstraZeneca-Oxford vaccine was approved for emergency use by the UK health regulator on 30 December 2020 and will be available for use in Africa once regulators on the continent approve it. It is likely that the approval will be obtained in the first quarter of 2021. This vaccine was also tested in parts of the continent like South Africa.
At the time of writing, South Africa had secured 1.5 million doses of AstraZeneca-Oxford vaccine from the Serum Institute of India which is producing these vaccines for low- and middle-income countries. The national regulator is in the process of reviewing the relevant vaccine data in order to grant approval for its emergency use in the country.
The African Vaccine Acquisition Task Team established by African Union Chair President Cyril Ramaphosa recently secured a provisional 270 million vaccine doses for African countries. The vaccines will be supplied by AstraZeneca-Oxford, Pfizer-BioNTech and Johnson & Johnson. The distribution of these vaccines on the continent will likely depend on the population size of each country. Even with additional 600 million vaccine doses promised by the World Health Organisation’s COVAX facility, the cumulative total vaccine doses available will only be enough for about half of the continent’s population.
Another vaccine that is likely to be available to the continent soon is that manufactured by Moderna. Pfizer-BioNTech and Moderna vaccines have already been approved for emergency use by the US as well as UK health regulators.
What about offerings from Russia and China?
The Gamaleya Research Institute of Epidemiology and Microbiology in Russia and Sinopharm in China allowed the public use of their vaccines before conducting large-scale clinical trials. This raised huge public concerns.
Conducting clinical trials and openly sharing the data from the trials is the international standard practice. The practice is to conduct three phases, all with smaller scale tests at first. Results from such trials are reviewed to assess safety and efficacy. This always precedes regulatory approval. If this route isn’t followed it is unlikely these vaccines will get approval from the national health regulators as there is no data to review.
Nevertheless, countries are buying vaccines from China. The Financial Times is also reporting that countries are buying Russia’s Sputnik V vaccine while the two leading Chinese manufacturers, Sinopharm and Sinovac Biotech, have signed deals with more than a dozen countries.
But public safety and scrutiny is critical. Without data from the trials it will be difficult to get the public on board. Sinopharm has submitted clinical data to the World Health Organisation for review.
Which will be easiest to deliver in developing countries?
When Pfizer-BioNTech announced that their vaccine was 95% effective, they also said it had to be stored at a temperature of -70℃. Most low-income countries do not have the necessary cold chain infrastructure for storing and distributing this vaccine.
Vaccines that require standard refrigeration conditions, like many of those used in existing immunisation programmes in these countries, would be easiest to deliver. The AstraZeneca-Oxford vaccine is one.
Johnson & Johnson has also developed a vaccine being trialled in Africa. The phase III results for this vaccine will be available in February 2021. If the vaccine is proven to be safe and effective, it will be the easiest to distribute as it requires the standard refrigeration. And more importantly, it requires just one dose, which is logistically easier to deliver.
Will new COVID-19 variants affect roll-out plans?
At present, it is thought that the mutations will not affect the efficacy of the vaccines. But more data is still required to be sure about this. Scientists and vaccine manufacturers are assessing if the current vaccines are effective against the new variants. Preliminary data are encouraging.
The roll-out is therefore continuing and tests will continue to assess if the emerging variants affect the vaccines’ efficacy.
Why there is so much concern about how quickly the vaccines were developed?
There is a lot of misinformation and incorrect messaging on social media platforms about the new vaccines. One incorrect message is that the vaccines were short-circuited and that this could have compromised them. It is important to emphasise that due diligence was adhered to and no short cuts were taken. These vaccines were tested properly and are proved to be safe. Additional monitoring of safety is ongoing as the vaccines get rolled out to more people following approval by the regulators. Safety monitoring will remain a key component of the vaccines roll-out.![]()
Benjamin Kagina, Senior Research Officer, Vaccines For Africa Initiative, Faculty of Health Sciences, University of Cape Town.
UCT’s response to COVID-19 in 2021
COVID-19 is a global pandemic that caused President Cyril Ramaphosa to declare a national disaster in South Africa on 15 March 2020 and to implement a national lockdown from 26 March 2020.
UCT is taking the threat of infection in our university community extremely seriously, and this page will be updated regularly with the latest COVID-19 information. Please note that the information on this page is subject to change depending on current lockdown regulations.

Global Citizen Asks: Are COVID-19 Vaccines Safe & Effective?
UCT’s Institute of Infectious Disease and Molecular Medicine (IDM) collaborated with Global Citizen, speaking to trusted experts to dispel vaccine misinformation.
If you have further questions about the COVID-19 vaccine check out the FAQ produced by the Desmond Tutu Health Foundation (DTHF). The DTHF has developed a dedicated chat function where you can ask your vaccine-related questions on the bottom right hand corner of the website.
IDM YouTube channel | IDM website
UCT Community of Hope Vaccination Centre
The University of Cape Town in partnership with the Western Cape Government (WCG) have reinforced our commitment to bringing hope to the residents of the Mother City with the launch of the world‑class Community of Hope Vaccination Centre that opened its doors on Monday, 30 August 2021.
The site is located on Main Road in Mowbray – in the Forest Hill Residence – and access is from Broad Street. The site is open every Monday to Friday from 08:00 to 15:00 and on Saturday from 09:00 to 13:00. Please allow time for attending to COVID-19 protocols and arrive as early as possible at the vaccination centre.
Frequently asked questions
News and views
Campus communications
2021
Media releases
Read more
UCT statements related to COVID-19 vaccinations
This is a space created for all formal bodies and structures within the university community to share their opinions on the need for a mandatory COVID-19 vaccine policy. Please note that some editorial judgement may be applied if the received statements go against any constitutional rights, and that no correspondence will be entered into, statements will be posted unedited and as received. Statements can be sent to opinions@uct.ac.za.
Commemorating a year of COVID-19
At midnight on 26 March 2020, South Africa went into the first nationwide hard lockdown. A year later, we remember those who have died and those who have been affected by COVID-19, as well as the pandemic’s effects across society and campus. We are especially grateful for the front-line health workers who have done so much for so many.
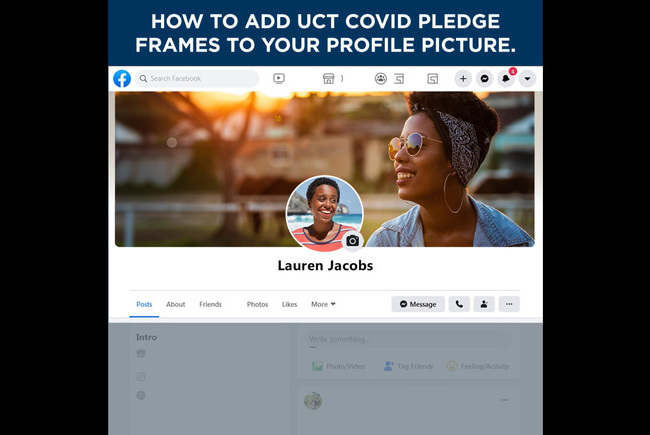
#UCTCOVIDPledge – social media elements
Customised Facebook frames and Instagram stickers are now available on those social media platforms. Watch the tutorial videos here to see how easily you can show your support for the #UCTCOVIDPledge.
In an email to the UCT community, Vice-Chancellor Professor Mamokgethi Phakeng said:
“COVID-19, caused by the virus SARS-CoV-2, is a rapidly changing epidemic. [...] Information [...] will be updated as and when new information becomes available.”
We are continuing to monitor the situation and we will be updating the UCT community regularly – as and when there are further updates. If you are concerned or need more information, students can contact the Student Wellness Service on 021 650 5620 or 021 650 1271 (after hours), while staff can contact 021 650 5685.