Valve revolutionises heart surgery
04 July 2012 | Story by Newsroom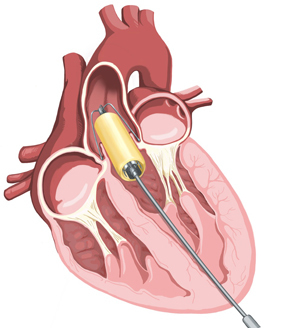

Research at UCT into the development and manufacture of devices that can replace damaged heart valves without the need of expensive open-heart surgery is attracting the right sort of interest.
The project has already sparked the formation of two new biotechnology companies - Southern Access Technologies Holdings (SATH) and its subsidiary, Southern Access Technologies (SAT) - which together have drawn investments totalling R30 million. SATH has raised R18 million from the Bidvest Group, while SAT has been granted another R12 million by the national Technology Innovation Agency.
The funds will be used to develop a new technique to assist victims of rheumatic heart disease, which is estimated to affect up to 78 million people worldwide, particularly in emerging and developing countries.
The project will tackle heart-valve diseases through the development of devices that can be deployed under conditions prevalent in developing countries and emerging economies, without requiring open-heart surgery and sophisticated operating theatres.
SATH's heart valve technology is based on research conducted at UCT, which is a major shareholder in the company. The company will begin immediately to develop prototypes for a heart valve, a delivery device for trans-catheter heart valves, and a clip device for the repair of heart valves.
The SATH products will be available at substantially lower cost than those of commercially available catheter-delivered valves.
Catheter-based heart-valve replacement has fast gained ground in the treatment of very old, inoperable patients with degenerative heart disease in the developed world, says Zilla. Now the rest of the world can also benefit from this procedure.
"Counterintuitively, this latest technology holds the key for the millions of patients in developing countries that have no access to open heart surgery," says Zilla.
The trick has been to find local alternatives to what's available in the Europe and North America, he explains. This includes the use of synthetic materials for the heart valve, sidestepping the "monopoly" on treated animal tissue traditionally used to produce such valves in developed countries, and so cutting the price by some margin.
In addition, the procedure to place the valves will additionally be substantially cheaper, most notably because open heart surgery will not be required. The therapies, Zilla explains, can be conducted in secondary hospitals that now lack the technology needed to treat the same heart condition, thus opening up new markets for heart valve products.
"Through UCT's intellectual property, the placement will be possible under secondary hospital conditions undercutting the expenses of sophisticated infrastructure and specialist expertise," says Zilla.
Testing of the products is expected to begin in late 2013.
SAT was founded by Professor Peter Zilla, head of UCT's Christiaan Barnard Department of Cardiothoracic Surgery at Groote Schuur Hospital and the Red Cross War Memorial Children's Hospital in Cape Town; Dr Deon Bezuidenhout, a polymer scientist who specialises in the development of biomaterial scaffolds and biomimetic matrices for tissue engineering and regeneration applications at UCT; and Professor David Williams, one of the world's leading experts in biomaterials and implantable medical devices.
The new technology will add to the Western Cape's growing reputation as a hub for innovative medical-device manufacture. Dr Greg Starke, a UCT biomedical engineering graduate who started stent manufacturer Disa Vascular, and Dr Kit Vaughan, the CEO of mammography equipment manufacturer CapeRay, are members of the SAT board of directors.
"There is considerable synergy between SAT and many of UCT's initiatives around healthcare for South Africa, which will provide key contacts to other African countries," said Professor Danie Visser, deputy vice-chancellor responsible for research at UCT. "This technology is a perfect example of the kind of socially responsive research that is a strategic goal of UCT.
 This work is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License.
Please view the republishing articles page for more information.










