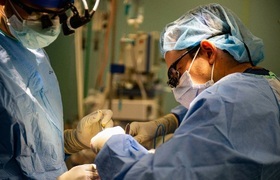
UCT breathes LIFE into ventilator solution
28 July 2020 | Story Nadia Krige. Photo Supplied. Voice Lerato Molale. Read time 8 min.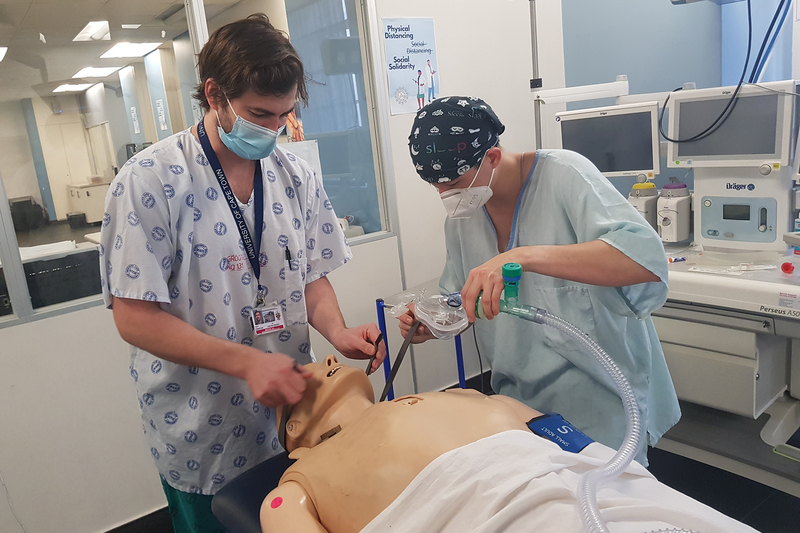
The University of Cape Town’s (UCT) Medical Devices Lab has played an integral role in developing a locally manufactured ventilator that has been approved for nationwide rollout to support patients showing respiratory distress in the early phase of COVID-19 infection.
The device forms part of the National Ventilator Project (NVP), which was launched in April by the South African Department of Trade, Industry and Competition as part of the government’s strategy to combat the coronavirus outbreak, and was designed by the Council for Scientific and Industrial Research (CSIR) in collaboration with local partners.
The goal of the NVP is to roll out 10 000 ventilators by the end of September 2020 – South Africa’s expected COVID-19 peak. According to the CSIR, the first batch will be ready for delivery by the end of July, and underresourced state hospitals will be prioritised.
UCT’s Medical Devices Lab – one of the partners – provided the CSIR with invaluable clinical input, including the all-important task of conducting usability tests.
“We’re so excited about the fact that the work that happens in a small lab on top of [UCT’s] Anatomy Building is now going to impact tens of thousands of people in South Africa at a time when it is needed most,” says Dr Sudesh Sivarasu, associate professor of biomedical engineering in the UCT Department of Human Biology, Faculty of Health Sciences, and head of the Medical Devices Lab.
“If this can help to save one person’s life, that’s massive.”
The ventilator, named CSIR LIFE (Lung Inspiratory Flow Enabler), uses standard, hospital-grade oxygen supply to provide a mild level of oxygenated air pressure that helps keep patients’ airways open.
Crafting a fool-proof design
Before being rolled out to clinical settings, medical devices like this need to be registered with the South African Health Products Regulatory Authority (SAHPRA). And to be successful, the inventors need to demonstrate that their device is safe and won’t introduce any harm to end-users.
It’s important that medical devices are intuitive to use, as this goes a long way in mitigating errors in the way the device is used and subsequent harm to the patient. As such, usability testing is an integral part of the design process.
Once UCT’s usability tests had been conducted, the CSIR LIFE system was submitted to SAHPRA and subsequently successfully registered.
Dedication of two young engineers
What exactly does usability testing entail, particularly for a medical device that could save thousands of lives?
In the case of CSIR LIFE, the quick answer is: days and nights of hard work and dedication by two young researchers.
Mechatronics engineer Edmund Wessels – currently completing his PhD in biomedical engineering – and mechanical engineer Catherine Gordon-Grant – currently completing her master’s in biomedical engineering – have been working tirelessly on the project since well before lockdown. They are striving towards a ventilator design that’s the best fit for the South African clinical environment.
Their journey started with the OpenAir Ventilator – a simple, low-cost solution using off-the-shelf products that was also entered into the NVP bid (with the CSIR as the manufacturing partner). Even though it was unsuccessful, the knowledge they gained in their own design process contributed to the team’s success as clinical partner for the CSIR’s successful bid.
“The purpose of our usability testing is to ensure that the devices are as intuitive and safe as possible to ensure that there aren’t any risks in a real-life situation,” says Gordon-Grant.
The usability testing that Wessels and Gordon-Grant led required hours of observing clinicians working with the ventilator in an environment simulating real life as closely as possible.
As they observed the clinicians handling the ventilator in these test situations, Wessels and Gordon-Grant broke down the sequence of events into individual tasks so that they could identify the exact points that posed difficulties.
“This is where a biomedical engineer’s role becomes really important: understanding how to meet those medical needs and how to translate them into engineering specifications – bridging the gap between these two parties,” says Wessels.
The main usability concerns were collected in a comprehensive report for the CSIR. Based on these findings, the CSIR then adapted their design. The report also formed part of the application sent to SAPHRA to show that the system had been thoroughly fault-checked.
Multi-faculty collaboration
Sivarasu, Wessels and Gordon-Grant say that throughout the process, they were humbled by the voluntary participation and support from various teams at UCT.
“One of our prominent collaborators has been the Department of Anaesthesiology,” says Sivarasu. “There are several doctors there who have tirelessly helped us through this process. We’ve been really privileged to have their input.”
“The purpose of our usability testing is to ensure that the devices are as intuitive and safe as possible…”
The Clinical Skills Centre also made a major contribution by offering the team the use of their facilities and dummies. Both the Clinical Research Centre and the office for Research Contracts and Innovations played a major role in guiding the administration process.
Finally, the Departments of Electrical and Mechanical Engineering offered invaluable input on the more nitty-gritty aspects of the design.
“It was really a multi-faculty collaboration,” says Sivarasu.
Future opportunities
The Medical Devices Lab has also been recognised as the only service provider to perform such ventilator usability trials in the country.
“We have five additional companies who are lined up to do the ventilator usability trial we did for the CSIR,” says Sivarasu.
Of course, this expands the impact of UCT during this time of global crisis.
“It’s not just about 10 000 ventilators,” says Sivarasu. “It’s about all those 10 000 ventilators being locally manufactured by local engineers. It’s the local job creation and local impact and innovation and that has more value than the actual numbers.
“Beyond that, if this can help to save one person’s life, that’s massive.”
 This work is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License.
Please view the republishing articles page for more information.