Educating people on TB patients’ journeys, and profiling the team behind the vaccine
25 March 2022 | Words Supplied. Photos Lerato Maduna.The University of Cape Town (UCT) commemorated World Tuberculosis (TB) Day on March 24 to raise public awareness about the devastating health, social and economic consequences of TB, research done and to step up efforts to end the global TB epidemic. TB remains one of the world’s deadliest infectious disease killers.
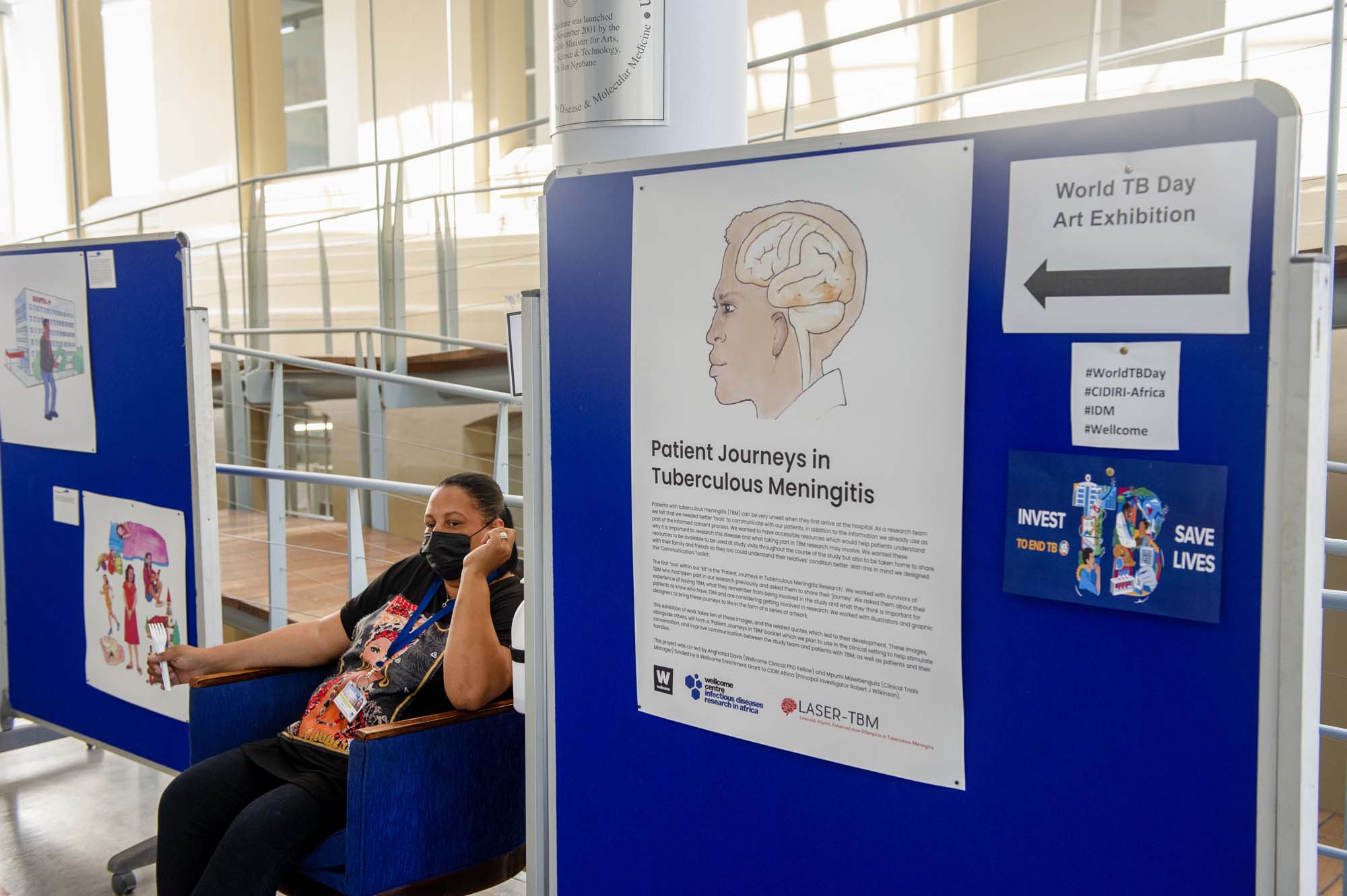
This week (22 to 25 March), UCT’s Wellcome Centre for Infectious Diseases Research in Africa (CIDRI-Africa) hosted an art exhibition at the Institute of Infectious Disease & Molecular Medicine (IDM), situated in the Wolfson Pavilion Building, in the Faculty of Health Sciences as part of efforts to educate researchers and the wider public about the patient journey in tuberculous meningitis research. The exhibition displayed images from the Patient Journeys in Tuberculous Meningitis Research project, co-led by Angharad Davis (Wellcome clinical PhD fellow) and Mpumi Maxebengula (study coordinator).
The project stems from the need to develop tools to better communicate with patients involved in TB research, in addition to the information researchers already use as part of the informed consent process, which would help patients understand why it is important to research this disease and what taking part in TB research may involve.
Davis and Maxebengula worked with illustrators and graphic designers to bring these patient journeys to life in the form of a series of artwork to share with their family and friends so they too could understand their relatives' condition better.
Recently, the South African Tuberculosis Vaccine Initiative (SATVI), a world leader in TB vaccine clinical research located within the IDM’s Health Sciences Faculty at UCT, captured images profiling their project team.
SATVI conducts TB vaccine clinical trials, and other general trials, which ensures the safety and effectiveness of new drugs or vaccines for treating, curing or preventing diseases. Clinical trials are conducted in phases amongst small groups of people in the early Phases (Phases I and II) and amongst larger groups of people once safety has been determined (Phases III and IV).
 This work is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License.
Please view the republishing articles page for more information.