Genetic detective: case of the neural crest
18 October 2019 | Story Ambre Nicolson. Photo Dorit Hockman. Read time 8 min.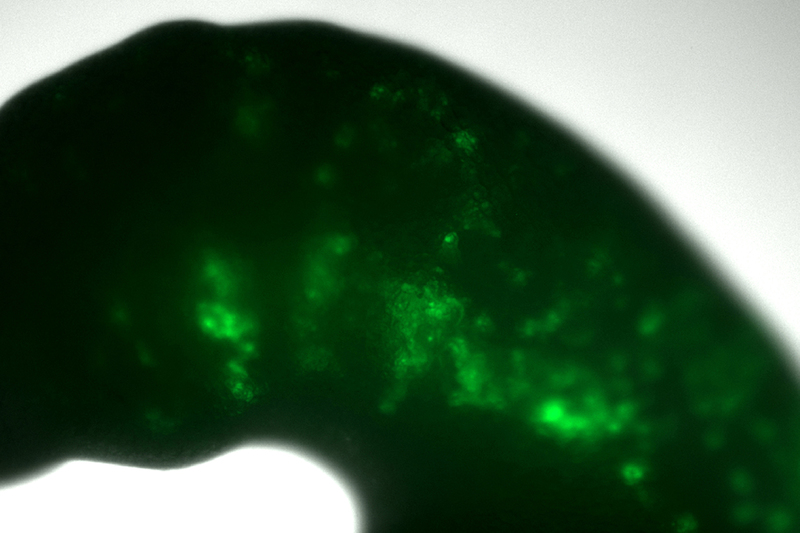
A new paper published by Dr Dorit Hockman, a developmental biologist based at the University of Cape Town (UCT), uses the most primitive living vertebrate – the lamprey – as a genetic time machine.
She explains how studying a specialised group of cells called the neural crest can help us understand the development of vertebrates – including ourselves.
What lampreys and humans have in common
Hockman, an evolutionary developmental biologist, first came into contact with lampreys as part of her PhD studies at Cambridge University.
“These small, jawless eel-like creatures are very useful organisms to study to better understand vertebrate evolution because they are at the evolutionary base of vertebrates,” she explains.
In humans, disruptions in neural crest development account for up to one-third of congenital birth defects, such as cleft lip and palate
“In other words, we assume that the lampreys of today look quite similar to some of the earliest vertebrates that existed.”
This also means that lampreys have neural crest cells, a group of cells found only in vertebrate embryos. These cells originate near an embryo’s brain and migrate to form critical parts of the adult body, including the skull and peripheral nervous system. In humans, disruptions in neural crest development account for up to one-third of congenital birth defects, such as cleft lip and palate.
It is thought that the evolution of the neural crest is one reason why vertebrates were so successful in evolutionary terms.
“This powerful cell population can become a lot of different kinds of tissues and structures, from neurons to teeth, cartilage and skin pigmentation. The neural crest explains the great diversity of vertebrate organisms.”
By studying the manner in which the lamprey’s neural crest develops, Hockman and her co-researchers were able to create a genetic database which can be compared to other vertebrates to better understand what parts of the neural crest are conserved back through time – all the way to the beginning of the vertebrate family tree.
“In simple terms, we were looking for the ‘core recipe’ of the neural crest.
“The neural crest explains the great diversity of vertebrate organisms.”
“The lamprey, being a basal vertebrate, is a good candidate for this since it has one of the most basic versions of the neural crest still in existence,” Hockman says.
The SoxE1 surprise
In searching for these essential elements that make up the neural crest, Hockman and her team used novel genomic approaches to analyse the genetics behind neural crest development in the lamprey.
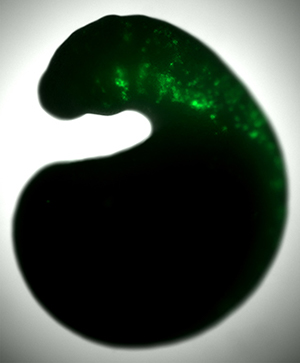
“One technique which proved very rewarding was studying the chromatin accessibility of lamprey neural crest cells,” Hockman explains. Chromatin accessibility describes the way DNA, which is very tightly coiled, unwinds – or opens – so that proteins can reach and activate its genes.
“By isolating these open regions, you can create sequencing libraries from which you can identify these active regions”.
Hockman does this by dissecting groups of cells from the lamprey and then extracting their nuclei – the bits of cell that house most of its DNA. She then exposes their DNA to an enzyme called a transposase, which nips any stretches of DNA that are open and inserts adaptors, which she describes as “like little tags”.
The researchers can then use the tags to copy and amplify the regions of the DNA that are open: “a bit like the copy and paste function on your computer, says Hockman. “This then allows us to map them to the genome.”
Hockman used this technique to identify the switches that turn important neural crest genes, such as SoxE1, on and off.
“We knew SoxE1 was an essential ingredient to the core recipe of the neural crest, but we wanted to know if the process of turning this gene on was conserved among later vertebrates.”
To explore this idea, the team turned to the embryos of more recently evolved vertebrates: chicken and zebrafish. They began by putting the lamprey genetic switch for SoxE1 into these animals’ DNA, linked to green fluorescent protein – which, as the name suggests, shines green when exposed to light.
By observing the modified embryos, they could deduce whether and where the switch was being used in developing chicken and zebrafish. Where the cells turned green, it would mean the lamprey switch for the SoxE1 gene was being turned on.
“People assume that the defining characteristic of a vertebrate is having a spinal column, but in fact, it is the neural crest that defines being a vertebrate.”
“We could see, much to our surprise, that the switches were in fact turned on in zebrafish and chicken embryos. And they switched on the equivalent region that they were switched on in the lamprey.”
A genome-wide first
There are two main reasons why this research, published this week in Nature Communications, is important.
“People assume that the defining characteristic of a vertebrate is having a spinal column, but in fact, it is the neural crest that defines being a vertebrate. There is great value in understanding how this evolved,” explains Hockman.
“Since the neural crest gives rise to so many things, it is often linked to congenital abnormalities. Understanding which genes and genomic switches are most important in the neural crest would therefore be useful to know from a medical point of view,” explains Hockman.
In the past, genetic studies of the lamprey mostly took the approach of studying candidate genes in which individual genes deemed likely to be important were studied one by one.
“This approach is now being replaced by big-data mining in which we can study many genes at once.
“This paper is the first time that we have a genome-wide assessment of gene expression in the neural crest in the lamprey as well as the first database that includes the non-coding elements or switches that are turning these genes on.
“Our hope is that it provides a useful means of comparison in the future.”
- Hockman D et al. (2019) A genome-wide assessment of the ancestral neural crest gene regulatory network. Nature Communications 10: 4689. https://doi.org/10.1038/s41467-019-12687-4
 This work is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License.
Please view the republishing articles page for more information.
Research & innovation





































