Cancer treatment gets personal
14 January 2020 | Story Krupa Naran, Dharanidharan Ramamurthy, Neelakshi Mungra and Prof. Stefan Barth. Photo Nephron, Wikimedia. Read time 7 min.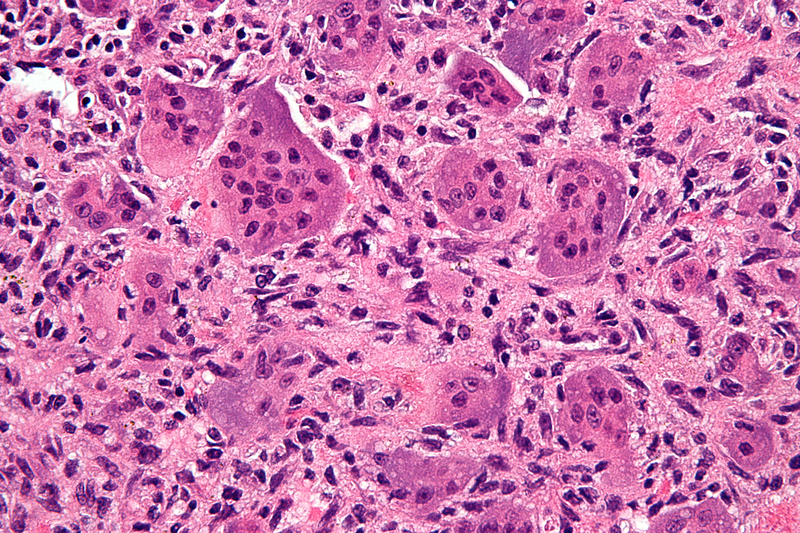
The search for better cancer treatments continues, as current options often cause severe side effects. Less than 5% of experimental anticancer drugs are approved for use in humans, but scientists are bringing new technologies to the quest.
Cancer is caused by multiple contributing factors. These include genetic mutations and abnormalities which cause cells to grow faster than normal. The differences between normal and tumour cells have started to become clearer to researchers since the human genome was mapped. It is now understood that a single approach to cancer treatment is inadequate. There is a need for more targeted and personalised therapies.
It is now understood that a single approach to cancer treatment is inadequate. There is a need for more targeted and personalised therapies.
Precision medicine, as these therapies are known, uses information about a person to determine what strategies can better prevent or treat a condition. The information includes genetic patterns, lifestyle, metabolism, environment and cultural factors. It aims to make the treatment of cancer smarter by customising the drugs for the specific patient. This is also known as personalised medicine.
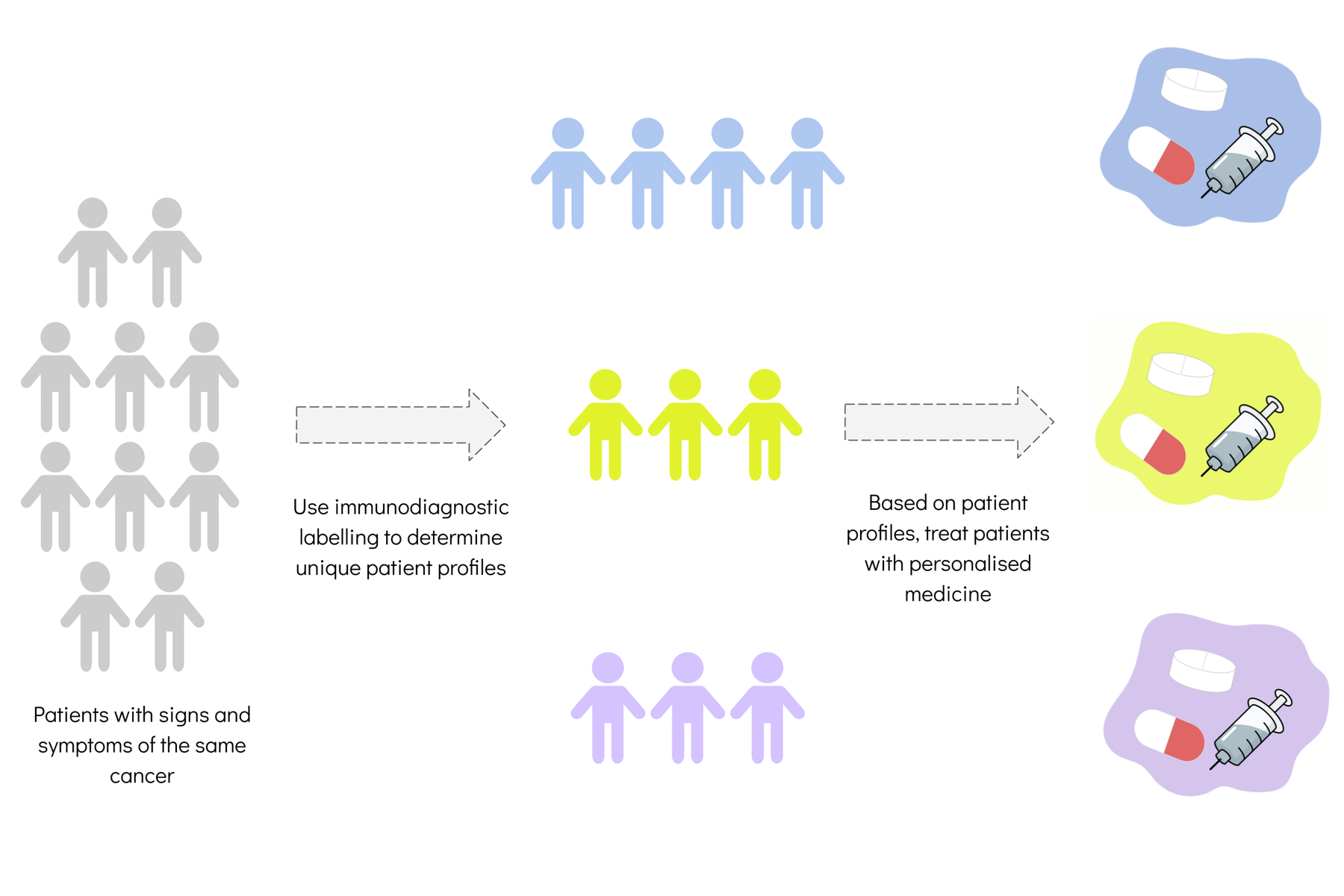
The concept of precision medicine is not new. In 2015, US president Barack Obama launched the Precision Medicine Initiative with the hope of making it part of routine patient care. But wider access to precision medicine requires cost-effective molecular profiling tests. An example is Oncotype DX, a genomic test that predicts breast cancer recurrence and response to treatment.
In several parts of the world, the search for precision medicine is using artificial intelligence and bioinformatic tools. These are computer software programs that analyse biochemical and biological information from ever-growing patient databases. The aim is to prevent harmful drug interactions, assess treatment efficacy and reduce the costs of health care.
The trend has also prompted South African research in the area of antibody technologies.
A team of researchers at the Medical Biotechnology and Immunotherapy Research Unit at the University of Cape Town has published a paper that explains how to generate certain proteins based on antibodies. Known as SNAP-tag fusion proteins, they may help reduce the cost and time it takes to develop new personalised therapies.
To understand this research, it’s necessary to know something about antibodies.
Antibody-guided precision medicine
Precision medicine is turning to antibody technologies to help identify important proteins (or biomarkers) that play a crucial role in disease.
Personalised cancer treatments such as immunotherapies are currently being used in combination with routine approaches like chemotherapy and surgery.
Antibodies are proteins normally produced by the body to guard against foreign substances such as disease-causing bacteria and viruses. They have unique characteristics such as the ability to recognise and bind with specific molecules (called antigens). Antibodies have so many potential uses in medicine that the global sales revenue for this market is expected to be US$218.97 billion by 2023.
The fact that antibodies have such specific targets is important in the development of targeted therapies known as immunotherapies. Antibodies can differentiate the body’s normal cells from those that are diseased (cancerous) based on the biomarkers expressed by the cells. Biomarkers are biological molecules and their detection determines the risk or progression of a particular disease.
By rearranging genetic material such as antibody genes, scientists can now generate new combinations of proteins for various applications. One example in precision medicine is the use of antibody technologies to identify breast cancer patients for treatment with trastuzumab.
SNAP-tag technology
In recent years, antibody engineering has been revolutionised by SNAP-tag technology. A SNAP-tag is a protein that can be fused to any molecule. It can form a link between antibodies that have recognised tumour cells and molecules that diagnose or treat the tumour. The SNAP-tag acts like the part of a key to which labels are attached. The lock is the biomarker on tumour cells, the key blade is the antibody, and the labels are the molecules used in diagnosis or treatment.
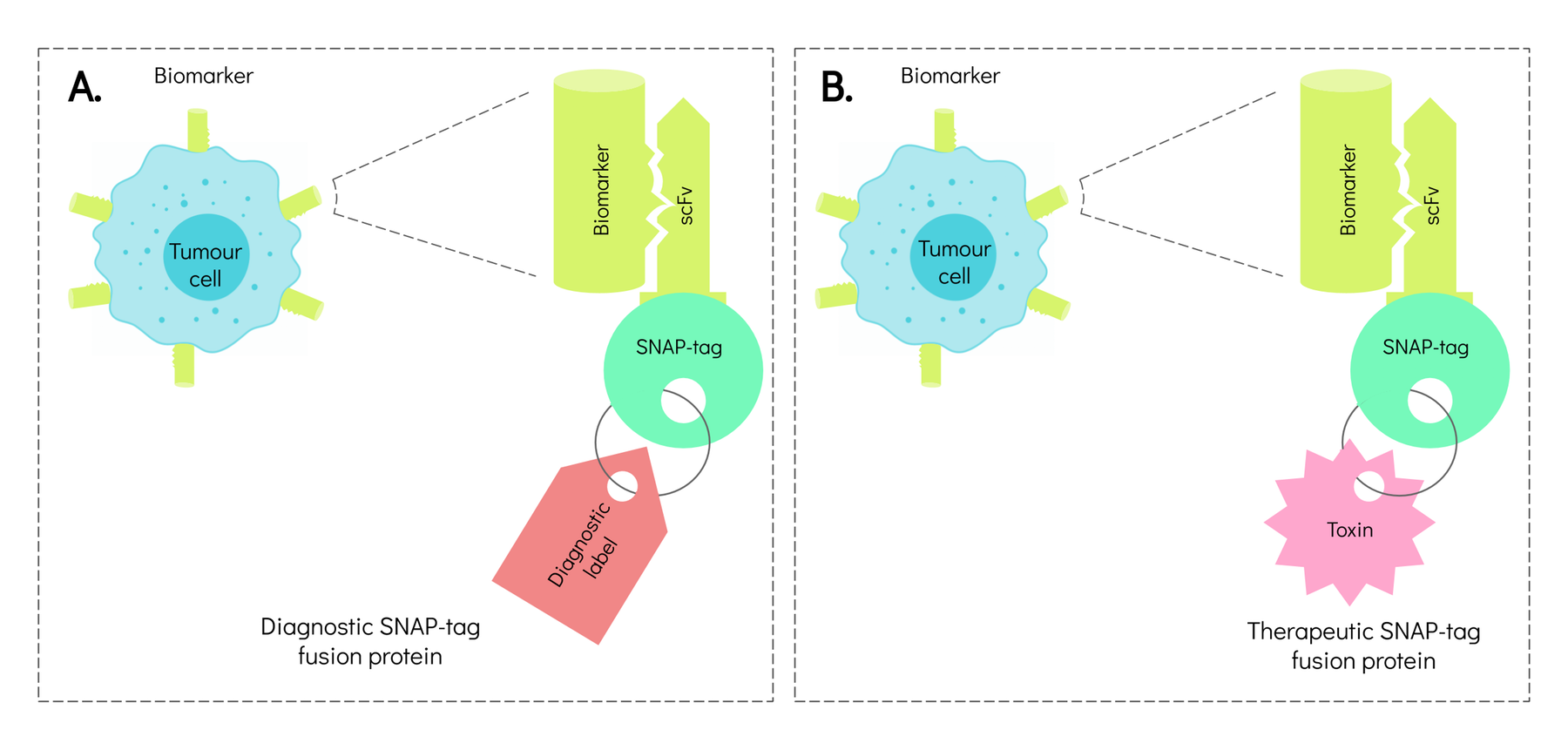
As a tool in precision medicine, antibody-based SNAP-tag proteins can identify patients who are likely to respond positively to a particular course of treatment. They can also reduce side effects and improve outcomes.
Future of antibody-based personalised medicine
The sooner oncologists are able to diagnose the type and stage of a cancer, the sooner the patient is able to begin the most suitable treatment. The development of rapid diagnostic tests could vastly improve patient survival. It could also limit side effects associated with damage to non-cancerous cells.
Personalised cancer treatments such as immunotherapies are currently being used in combination with routine approaches like chemotherapy and surgery. But even with reports of improved survival and quality of life in patients who respond to such treatments, they are expensive and not universally accessible.
Nonetheless, precision medicine is evolving and the search will continue for ways to make it more affordable, accessible and effective.![]()
Krupa Naran, Postdoctoral Research Fellow, Institute of Infectious Diseases and Molecular Medicine, University of Cape Town; Dharanidharan Ramamurthy, Postdoctoral research fellow, University of Cape Town; Neelakshi Mungra, PhD Candidate at the MB&I Unit, University of Cape Town, and Prof. Stefan Barth, South African Research Chair in Cancer Biotechnology (Tier 1).
Research & innovation





































