Leishmaniasis needs more attention
15 July 2019 | Story Ramona Hurdayal and Raphael Taiwo Aruleba. Photo James Gathany, Wikimedia. Read time 8 min.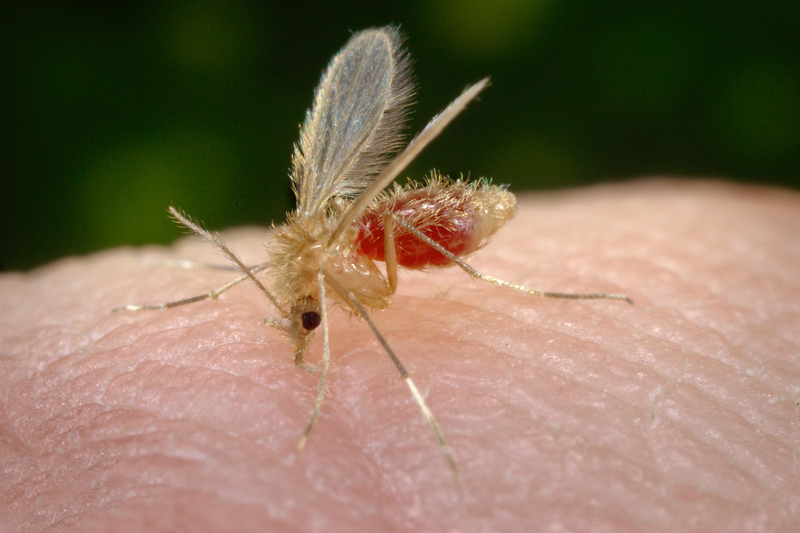
Leishmaniasis ranks on the World Health Organisation’s list of neglected tropical diseases, along with the usual suspects such as dengue, chikungunya and rabies.
But there’s a strong likelihood that it’s a disease you have never heard of. What’s even more surprising is that thousands of people in 89 countries across all continents, except Antarctica, die from the disease every year while an estimated 1.5 – 2 million are infected annually. Another 1 billion people are at high risk of infection.
Lesions can lead to disability and disfiguring scars – and stigma.
Like malaria, leishmaniasis is caused by a parasite that is carried by an insect – in this case, a female sandfly. After malaria it ranks as the second most deadly protozoan disease in humans. But, unlike mosquitoes that give just one form of malaria, the sandfly can give four forms of leishmaniasis. These forms range from lesions on the skin to infection of internal organs such as the spleen and liver. Lesions can lead to disability and disfiguring scars – and stigma. The other infections can be lethal if left untreated.
Leishmaniasis was first identified in the 1900s. Since then significant progress has been made in diagnosis, treatment and overall management. But, despite all efforts there’s still no vaccine. Which is why the WHO considers it a neglected disease of the world – under-funded and under-researched by both private and public organisations.
There is no convincing explanation as to why this is the case. One reason may be because it affects people who live in remote areas where reporting and diagnostic systems are poor and healthcare is a luxury.
The gap in knowledge – and treatment – of the disease is particularly bad for Africa. This is because the disease presents as an opportunistic infection in patients infected with HIV, TB and malaria. Sudan, for example, holds one of the highest rates of Leishmania and HIV co-infection. This co-infection is known as a “deadly gridlock” as both microbes strengthen each other. Leishmania infection accelerates the development of HIV to AIDS while HIV infection increases the risk of developing Leishmania infection between 100 to 2320 times.
But all is not lost. Several groups in different continents, including Africa, are working at understanding the complexities of this disease.
For example, we are part of a unit at the University of Cape Town that’s been shedding more light on the disease. In particular, we are analysing how host factors contribute to the rate of natural healing – or recurring infection, with the aim of targeting these for development as host-directed therapies.
Tackling a fatal disease
Leishmaniasis is medically complex and the fact that it “hides” in cells of the body makes treatment complicated. Sodium stibogluconate and liposomal amphotericin B are widely used to treat infection. But this involves a long duration of treatment that needs to be administered into the vein.
These drugs were introduced in the 1940s. Almost eighty years later they are still being used with only one other drug, Miltefosine, being approved in 2002.
Adding to this, new challenges have kept emerging. For example the efficacy of sodium stibogluconate is now being eroded by resistance. In India, for example, specifically Bihar, treatment failure rates have exceeded 60% since the 1990s and are known to induce toxicity. In addition, while liposomal amphotericin B deoxycholate has been shown to be highly effective, it’s expensive, which restricts its access to poor populations.
Leishmaniasis is medically complex and the fact that it “hides” in cells of the body makes treatment complicated.
This is why accessible and cheap high-quality medicine is important. The lack of a vaccine also complicates matters.
The hope is that our research will identify host factors that could be developed for alternative or complementary treatments, termed host-directed therapies, since an effective host response is required to support anti-leishmanial drugs.
Beyond the therapies, the next important area of work is integrating awareness to prevent and manage the spread of the disease.
Prevention better than cure
Mass drug administration, like that used for other tropical diseases such as schistosomiasis and malaria, is not recommended for leishmaniasis due to the invasive route of administration and associated drug toxicity. Thus, preventative measures instead must be strengthened. These include vector control – the use of pesticides and insecticides – social mobilisation to educate people in endemic areas on behavioural changes, treated nets and environmental management.
In addition, innovative ideas into alternate treatment options, such as host-directed therapies, must be explored.
In line with this, the WHO has targeted neglected diseases, including leishmaniasis, for control and elimination by 2030. This roadmap is being implemented to achieve the health-related 2030 United Nations Sustainable Development goals, together with policymakers, governments, NGOs, philanthropists, stakeholders, industries and essentially, the general public.
The starting point is that prevention is better than cure. Central to this theme is awareness – in endemic areas as well as more broadly. But, to build this awareness, we need a wider platform to reach a larger audience, both public and private.![]()
Dr Ramona Hurdayal, lecturer and team leader of the Leishmaniasis Research Group, University of Cape Town and Raphael Taiwo Aruleba, PhD candidate, University of Cape Town.










