Newly mapped ACE protein heralds new cardiovascular drugs
24 February 2003
Dedicated team: Dr Ed Sturrock (left) and his researchers (from left) Sylva Schwager, Zenda Woodman, Pierre Redelinghuys, Aloysius Nchinda and Aheska Parker, whose collaborative efforts with Bath University researchers have identified the first 3-D crystal structure of the human angiotensin-converting enzyme (ACE). Here they study a model of the ACE inhibitor, lisinopril.
TO THE eye, the magnified three-dimensional image of the human ACE protein may resemble an amorphous cluster shape of little significance. But to a UCT research team and their Bath collaborators, this newly mapped angiotensin-converting enzyme (ACE) holds the key to the development of new generation drugs for treating high blood pressure, myocardial infarction (heart attack), heart failure and a string of related ailments.
UCT researchers Dr Ed Sturrock and Sylva Schwager (Medicine and Clinical Laboratory Sciences) and their collaborators at Bath University, Professor Ravi Acharya and Dr Ramanathan Natesh, have determined the first 3-D crystal structure of ACE and its complex with one of the most widely used inhibitors, lisinopril. Lisinopril works by interacting with the ACE protein and causing widening of the blood vessels thus lowering the blood pressure and helping the heart to work more effectively.
The details of their work, which is supported by the Wellcome Trust (UK), appeared as an advance online publication in the prestigious Nature journal ( www.nature.com/ nature) and in the printed version on January 30.
ACE inhibitors are widely used to treat cardiovascular diseases, including hypertension, heart failure, coronary artery disease, diabetes and kidney failure. But current ACE inhibitors, developed in the 1970s and 1980s, produce common side effects. These include a persistent cough and angioedema (an allergic reaction causing swelling of the eyes, throat and tongue).
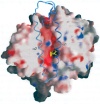 Up close: Molecular surface representation of the human angiotensin-converting enzyme (ACE). The ellipsoid shape of the ACE structure has a central groove extending into the molecule, dividing the protein into two sub-domains. The lisinopril molecule (the inhibitor) is shown in yellow.
Up close: Molecular surface representation of the human angiotensin-converting enzyme (ACE). The ellipsoid shape of the ACE structure has a central groove extending into the molecule, dividing the protein into two sub-domains. The lisinopril molecule (the inhibitor) is shown in yellow.
“ACE consists of two parts, the N and C domains, with different functions. Current drugs inhibit both domains,†Sturrock explained. “By designing specific domain-selective ACE inhibitors we expect to produce second-generation drugs that are safer and more effective and have fewer side effects.â€
Having determined the 3-D structure of ACE, researchers can begin synthesising new ACE inhibitors using structured structure-guided drug design. This process will involve and range of scientific input as computational chemists and structural biologists begin the complex molecular modeling process, using existing inhibitors as scaffolds on which to build new drug compounds. This work will be done in the UCT and Bath laboratories.
Novel C- and N-domain-selective inhibitors will then be evaluated for further development in partnership with pharmaceutical companies. AngioDesign, their “virtual†US-registered company, will explore the commercial potential attached to the design of new drugs www.AngioDesign.com).
“The potential is huge, but the time from breakthrough to presenting new drugs on the market is anything between six and 12 years,†Sturrock added. He and Acharya have taken out an international patent on their work, owned by both universities.
The UCT research group involves diverse disciplines and is part of the South African Structural Biology Initiative, (which kicked off this year with its first intake of six MSc students), which makes protein structure determination available in Africa for the first time. The research is unique in the sense that it covers a range of disciplines, from recombinant DNA technology (DNA that has been created artificially by “gene jockeysâ€) to structural biology and synthetic chemistry.
A UCT alumnus (after Honours he completed his PhD in 1994), Sturrock began work on the ACE protein nine years ago at Harvard Medical School while on a Fellowship sponsored by the National Research Foundation (NRF) and National Institutes of Health (NIH). It was at Harvard that he established links with his Bath collaborator.
After returning to South Africa, Sturrock set himself the goal of maintaining and developing his collaborations with leaders in this field abroad. “This was particularly important as the skills and technology in protein X-ray crystallography were lacking in South Africa and in the Western Cape,†he noted.
Solving the crystal structure of ACE has been a long haul and all-consuming work. “The main challenge is to get the protein to form tiny crystals and to make sure it is that they are suitable for X-ray diffraction studies,†he elaborated. “Once you have the crystals it is still a very technical and involved process to conduct the X-ray studies and solve the structure.â€
Publishing in Nature has certainly added to Sturrock's international reputation. “It's not an everyday occurrence,†he notes. “It's a scientist's dream, especially as very few Nature papers have been published by South African researchers.†As a result, he has been invited to deliver a talk at the International Protease Conference in Japan at the end of the year.
He is also hoping the recognition will act as a springboard to major advances, not only in understanding the ACE protein, but also its interaction with its inhibitors.
 This work is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License.
This work is licensed under a Creative Commons Attribution-NoDerivatives 4.0 International License.
Please view the republishing articles page for more information.
Related
InvestSoc at 25: advancing quality
27 Feb 2026
Prestigious global award for Prof Keertan Dheda
27 Feb 2026
NPO creates pipeline of empowered students
25 Feb 2026










